By using this site you agree to our use of cookies. Please refer to our privacy policy for more information. Close
CGMP controlled Raw Materials – Regulations and Best Practices
- By: Staff Editor
- Date: July 17, 2018
Compliance Webinars | Virtual Seminars for Professionals
CGMP controlled Raw Materials – Regulations and Best Practices
5155 Firms received FDA 483 between 10/1/2016 and 9/30/2017. One of the major reasons for issuing these FDAs was non-compliance with the regulatory requirements of cGMP controlled raw materials. It is critical for personnel in the Pharmaceutical, Biotechnology, and Medical Device Industries to understand the regulatory requirements and best practices pertaining to cGMP controlled raw materials to gain approvals and make products safe for use.
Regulations Guiding FDAs cGMP Controlled Raw Materials 
The Regulations Guiding cGMP Controlled Raw Material is in FDA regulations 21 CFR 211.84 Subpart E:;Testing and Approval or Rejection of Components, Drug Product Containers, and Closures.
The section D of this rule identifies what is needed to get drug products released and approved and also explains the basis on which products shall be rejected.
Other key points explained are:
- How to release each lot of components, drug product containers, and closures
- The basis on which the representative samples shall be collected for testing or examination
- What procedures should be followed for collection of samples with regard to cleaning containers, preventing contamination, sterile equipment aseptic sampling, sample containers identification, sub divisions and more
- How the samples will be examined and tested
You may also refer 21 CFR 211.80 for control of components and drug product containers and closers
21 CFR 211.110; In-process materials shall be tested for ID, strength, quality, and purity as appropriate, and approved or rejected by the quality control unit.
GMP Guidance B of VII 7.2. explains The process to follow on receipt and before acceptance of containers of materials
Other regulatory agencies that control regulate GMP.
ICH; in its Q7 code section 6.3 specifies what Records of Raw Materials, Intermediates, API Labelling should be documented.
European Medicines Agency GMP requires consistent high quality appropriate for their intended use and compliance with the marketing or clinical trial authorization. 
- Understand the regulatory requirements for all Incoming cGMP Controlled Raw Materials – mentioned under 21 CFR 211.84
- Follow Receipt and Storage of cGMP Controlled Raw Materials processes as per 21 CFR 211.82
- Process New cGMP Controlled Raw Material as per Specification and forward to Quality Control (QC) department for processing and testing.
- Complete a cGMP raw material request form to request the controlled material test specification and the raw material part number.
- Documents to include: The Raw Material Certificate of Analysis (C of A) that accompanied the raw material, and other applicable documentation (MSDS etc)
- Follow the Procedure for Raw Material Initial Receipt
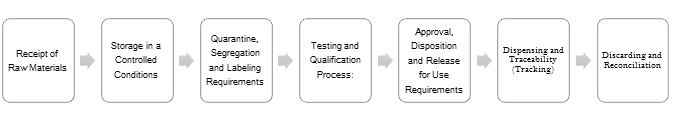
- Raw Materials are quarantined after initial receipt and verification process
- Store in a Controlled Conditions (Temperature and Humidity Control)
- Quarantine, Segregation and follow Labeling Requirements
- Perform the processing of the raw materials specification and testing
- Test Requirements for all Incoming cGMP Raw materials
- Establish a new part number for the cGMP controlled raw material.
- Perform an “Initial Qualification” testing of the new incoming raw material.
- Evaluate and institute an acceptable testing programs and frequency.
- For Routine ‘Qualified’ cGMP raw material a subsequent re-qualification test is required that confirms the previous qualification testing.
- Perform Routine and Yearly Confirmatory Testing for Suppliers and Manufacturers of Qualified cGMP Controlled Raw Controlled Materials
- Follow guidance for the industry section D. Control of Raw Materials (19.4)
Consumers highly regard quality products. It is key to understand and implement best practices, for life sciences companies and personnel which will pave the way for manufacturing and approval of high-quality products.
If you are a life sciences personnel, you can learn from experts to have a better understanding of the best practices and get the big picture of common practices followed in the industry. In the webinar titles CGMP controlled Raw Materials, panelist Charity Ogunsanya who is an authority in this subject has 23 years of experience will teach how to do confirmatory testing, how raw materials are issued and released and most importantly how to rule out lab errors through investigation process, the documentation requirement and many more areas. She will bring multiple scenarios based on her practical experience giving guidance to manage the activity efficiently. Click here to know more. .
Trending Compliance Trainings

By - Roger Cowan
On Demand Access Anytime

By - Doug Keipper
On Demand Access Anytime

By - Joy McElroy
On Demand Access Anytime

By - Carolyn Troiano
On Demand Access Anytime

By - Dr. Afsaneh Motamed Khorasani
On Demand Access Anytime



By - Michael Ferrante
On Demand Access Anytime


- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
By: Miles HutchinsonAdd to CartPrice: $249
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart
-
Add to CartSan Francisco, CA | Aug 6-7, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 18-19, 2020
-
Add to CartLos Angeles, CA | Aug 20-21, 2020
-
Add to CartVirtual Seminar | Jul 16-17, 2020
-
Add to CartVirtual Seminar | Jun 25-26, 2020
-
Add to CartVirtual Seminar | Jun 10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | Jul 6-7, 2020
-
Add to CartSan Francisco, CA | Oct 22-23, 2020
-
Add to CartVirtual Seminar | Jul 9-10, 2020
-
Add to CartVirtual Seminar | Jun 3-4, 2020
-
Add to CartVirtual Seminar | June 3-4, 2020
-
Add to CartMiami, FL | Jul 29-31, 2020
-
Add to CartVirtual Seminar | Jun 17, 2020
-
Provider: ANSIAdd to CartPrice: $142
- Add to Cart
- Add to Cart
- Add to Cart
-
Provider: ANSIAdd to CartPrice: $120
-
Provider: ANSIAdd to CartPrice: $250
-
Provider: SEPTAdd to CartPrice: $299
- Add to Cart
-
Provider: Quality-Control-PlanAdd to CartPrice: $37
- Add to Cart
-
Provider: At-PQCAdd to CartPrice: $397
- Add to Cart
- Add to Cart
- Add to Cart
- Add to Cart








