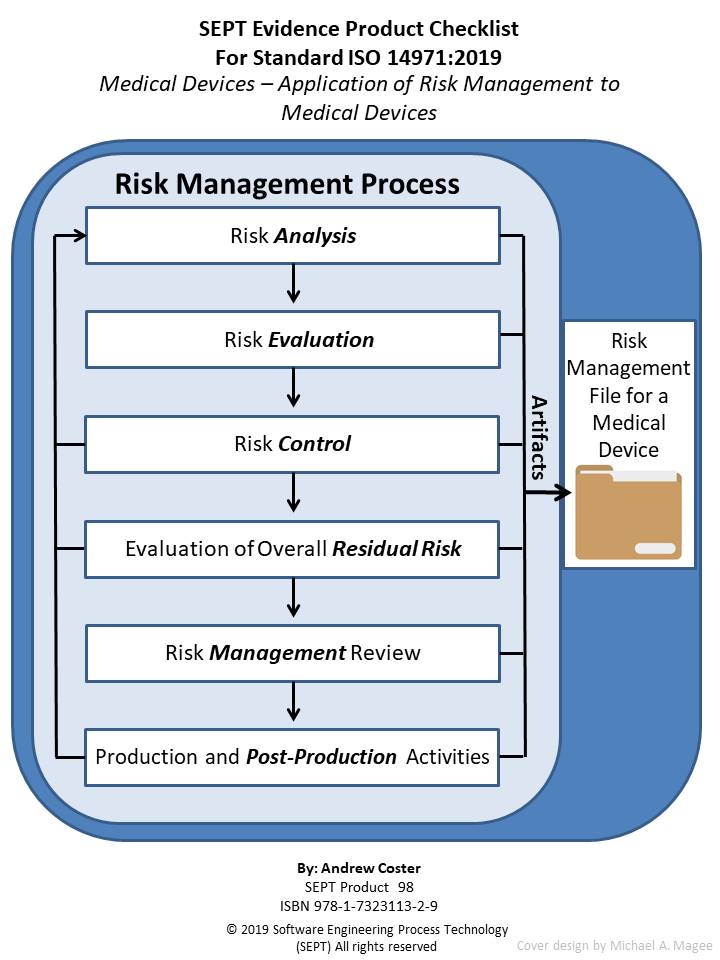
ISO 14971:2019 Medical devices - Application of Risk Management to Medical Devices
Author: Andy Coster, CCP
Cover: Available
Customer Set for this product: Medical Device Firms
Format : Word® (To save money, click here for our PDF version)
ISBN Numbers: ISBN 978-1-7323113-2-9
Language: English
Page count of document: 143
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
This is a checklist for ISO 14971:2019, another checklist related to medical device standards. The purpose of the checklist is to define clearly all the artifacts (policy, procedure, plan, records, document, or reviews) that the underlying standard calls out. Normally the SEPT checklist has a section for the artifact “audit”. However, the ISO 14971:2019 standard does not specify any requirements for Audits so this section is left blank for this checklist. Nevertheless, in many sections there is a requirement to inspect the risk management file. Furthermore, what constitutes physical evidence (Artifacts) to meet the guidance outlined in ISO 14971 is sometimes difficult to identify. To bridge this gap the author and SEPT experts have identified items of physical evidence called out in the standard based on their knowledge of the document and their experience in the standards field. Each item of physical evidence that was identified by these experts is listed in the checklist as an artifact (policy, procedure, plan, records, document, or reviews.)
There must be an accompanying record of some type when a review has been accomplished. This record would define the findings of the review and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review. All procedures should be reviewed but the checklist does not call out a review for each procedure, unless the standard calls out the procedure to be reviewed.
The author has carefully reviewed the Standard ISO 14971:2019 and defined the physical evidence required based upon this classification scheme. SEPT’s engineering department has conducted a second review of the complete list and baseline standard to ensure that the documents’ producers did not leave out a physical piece of evidence that a “reasonable person” would expect to find. If an artifact is called out more than one time, only the first reference is stipulated. If an artifact is required by ISO 14971:2019, it appears in the checklist without appended symbol. If an item is “suggested” either by ISO 14971:2019 or by inference when a document or plan is called out it appears with an appended asterisk (*). If an item is required to be included in the associated Risk Management File by ISO 14971:2019 it appears in the checklist with an appended symbol ( ). In this way traceability of requirements and suggested items as well as the need to include a required item in the Risk Management File is possible.
Note: These notations are listed in the footnotes for each section.
There are occasional situations in which a procedure or document is not necessarily separate and could be contained within another document. For example, the ‘Manufacturer Medical Device Reasonably Foreseeable Misuse Document’ could be a part of the ‘Manufacturer Medical Device Intended Use Document’. The author has called out these individual items separately to ensure that the organization does not overlook any facet of physical evidence. If the organization does not require a separate document, and an item can be a subset of another document or record, then this fact should be denoted in the detail section of the checklist for that item. This should be done in the form of a statement reflecting that the information for this document may be found in section XX of Document XYZ. If the organizational requirements do not call for this physical evidence for a project, this should also be denoted with a statement reflecting that this physical evidence is not required and why. The reasons for the evidence not being required should be clearly presented in this statement. Many of the procedures referenced could in fact be a part of a detailed Risk Management (Process) procedure. Further details on this step are provided in the Detail Steps section of the introduction. The size of these documents could vary from paragraphs to volumes depending upon the size and complexity of the project or business requirements.
These notations are listed in the footnotes for each section.
The checklist is available in PDF or word format. The latter format allows you to tailor the document to your business case or the media that your organization wants to use the checklist in such as excel web page format or any other end product type in order to meet compliance with the standard in the most efficient way possible.
The checklist comes with 4 hours of free consultation, from experts that have firsthand knowledge of the underlying standard, to answer questions on the standards and checklists and is valid for 60 days after purchase of the product.
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- Evidence Product Checklist For UL 1998 Standard for Safety - Software in Programmable Components
Price: $167 BUY NOW - ISO 17025 Quality Manual Template
Price: $299 BUY NOW - Requirements for Computer System Requirements, Validation Plans, Protocols and Reports (RiskVal)
Price: $99 BUY NOW - Change Control for Validated Systems (RiskVal)
Price: $125 BUY NOW - IT Policy (RiskVal)
Price: $99 BUY NOW - Validation Plan Template for Single System Validations (RiskVal)
Price: $99 BUY NOW