ISO/IEC 42010:2011 Systems and Software Engineering - Architecture Description
Author: Andy Coster, CCP and Stan Magee CCP (Ret.)
Cover: Available
Customer Set for this product: Software and System Engineering firms and company with large and complex software and systems
Format: Word® (To save money, click here for our PDF version)
ISBN numbers: 978-0-9819522-5-3/0-9819522-5-9
Language: English
Page count: 40
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
The ISO/IEC/IEEE 42010:2011 Systems and Software Engineering Architecture Description standard provides a core ontology for the description of architectures. The provisions of this standard serve to enforce desired properties of architecture descriptions. It also enforces desired properties of architecture frameworks and architecture description languages (ADLs) in order to usefully support the development and use of architecture descriptions. This standard provides a basis on which to compare and integrate architecture frameworks and ADLs by providing a common ontology for specifying their content.
This international standard can be used to establish a coherent practice for developing architecture descriptions, an architecture framework, and architecture description languages within the context of a life cycle and its processes. Conformance requirements for architecture frameworks and architecture description languages are provided in the standard to help insure the reusability and interoperability of architecture descriptions.
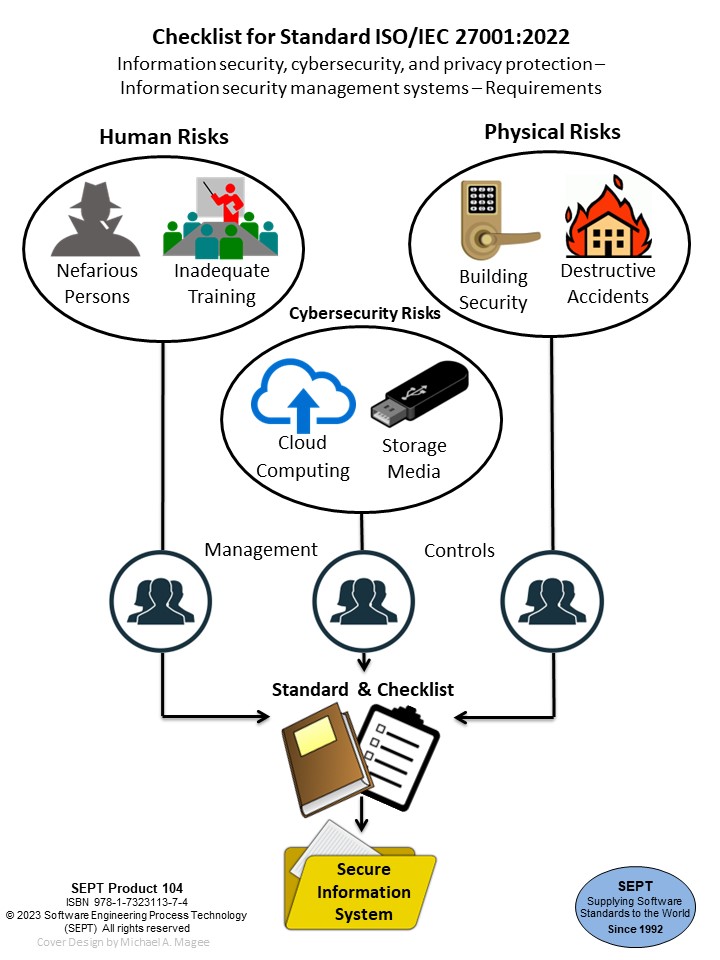
Now! The experts at SEPT have produced a checklist for ISO/IEC/IEEE 42010:2011. This checklist was prepared by analyzing each clause of this document for the key words that signify a policy, procedure, plan, record, document, audit, or review. The Checklist provides an easy-to-use classification scheme of physical evidence comprised of procedures, plans, records, documents, audits, and reviews. The numbers of these entities which are required or suggested by the standard are shown below.
Artifact |
# |
Policy and Procedure |
35+ |
Plan |
1 |
Record |
5 |
Document ( Including Manuals, Reports, Scripts and Specifications) |
15+ |
Audit |
2 |
Review Total |
15+ 70+ |
The Checklist clarifies what is required for compliance through a product evidence list that will assist any organization in meeting the requirements of this standard. Every Checklist comes with four hours of free consultation. SEPT will answer any question concerning the standard or Checklist for 60 days after purchase. Use of the Checklist will save time and money, and may aid in meeting certain governmental requirements. This is an aid that will pay dividends. A quality product at a reasonable price!
This product supports these Software Engineering processes
- Configuration Management
- Design
- Life Cycle
- Maintenance And Operation
- Metrics
Customers of this product:
- Hutchison Drei Austria GmbH, Vena Austria
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- ISO/IEC/IEE Standard 15288:2015-Systems and Software Engineering-System Life Cycle Processes - (Third Edition)
Price: $330 BUY NOW - A Software Engineering Kit – Composed of Templates for Key Software Engineering Process Plans
Price: $298 BUY NOW - ISO/IEC/IEE Standard 15288:2015-Systems and Software Engineering-System Life Cycle Processes - (Third Edition)
Price: $167 BUY NOW - Checklist for Standard ISO/IEC 27002:2013 - Information Security Code of Practice
Price: $167 BUY NOW - Template for a Software Maintenance Plan - Fifth Edition
Price: $149 BUY NOW - Evidence Product Checklist for ISO/IEC 12207:2017 ''System and Software Engineering - Software Life Cycle Processes''
Price: $167 BUY NOW