Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices As amended by Guidance for Industry, FDA Reviewers and Compliance on Cyber security for Networked Medical Devices Containing Off-the Shelf (OTS) Software"
Author: Stan Magee CCP (Ret.)
Cover: Available
Customer Set for this product: Medical Device Firms
Format: Word® (To save money, click here for our PDF version)
ISBN Numbers: 978-0-9748987-9-7 / 0-9748987-9-1
Language: English
Page count of document: 21
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
The experts at SEPT have updated Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices to reflect the suggested compliance with the document” Compliance on Cyersecurity for Networked Medical Devices Containing Off-the Shelf (OTS) Software dated January 14, 2005 This checklist reflects these new requirements. The Checklist provides an easy-to-use classification scheme of physical evidence comprised of procedures, plans, records, documents, audits, and reviews. The Checklist clarifies what is required for compliance through a product evidence list that will assist any software organization in meeting the requirements of this guideline. Every Checklist comes with four hours of free consultation. SEPT will answer any question concerning the standard or Checklist for 60 days after purchase. Use of the Checklist will save time and money, and may aid in meeting certain governmental requirements. A quality product at a reasonable price!
This product supports these Software Engineering processes
- Configuration Management
- Design
- Documentation
- Integration
- Life Cycle
- Quality
- Safety
- Security
- Verification And Validation
Customers of this product:
- Minerva Surgical
- Olympus software technology, Japan
- SCC Soft Computer
- Uptake Medical
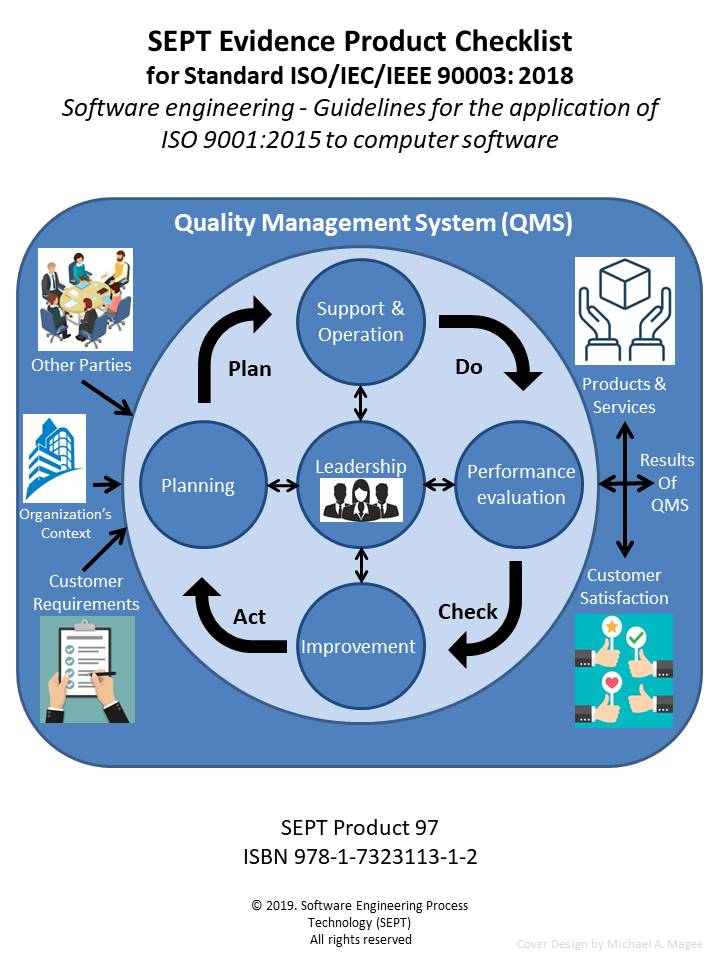
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- ISO 14971:2019 Medical devices - Application of Risk Management to Medical Devices
Price: $330 BUY NOW - Checklist for FDA, Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices.
Price: $330 BUY NOW - Electronic Signatures; Final Rule-FDA 21CFR Part 11
Price: $167 BUY NOW - FDA, General Principles of Software Validation Final Guidance for Industry and FDA Staff (Release date January 11, 2002)
Price: $167 BUY NOW - ISO 13485:2016 “Medical Devices - Quality Management Systems- Requirements for Regulatory Purposes”
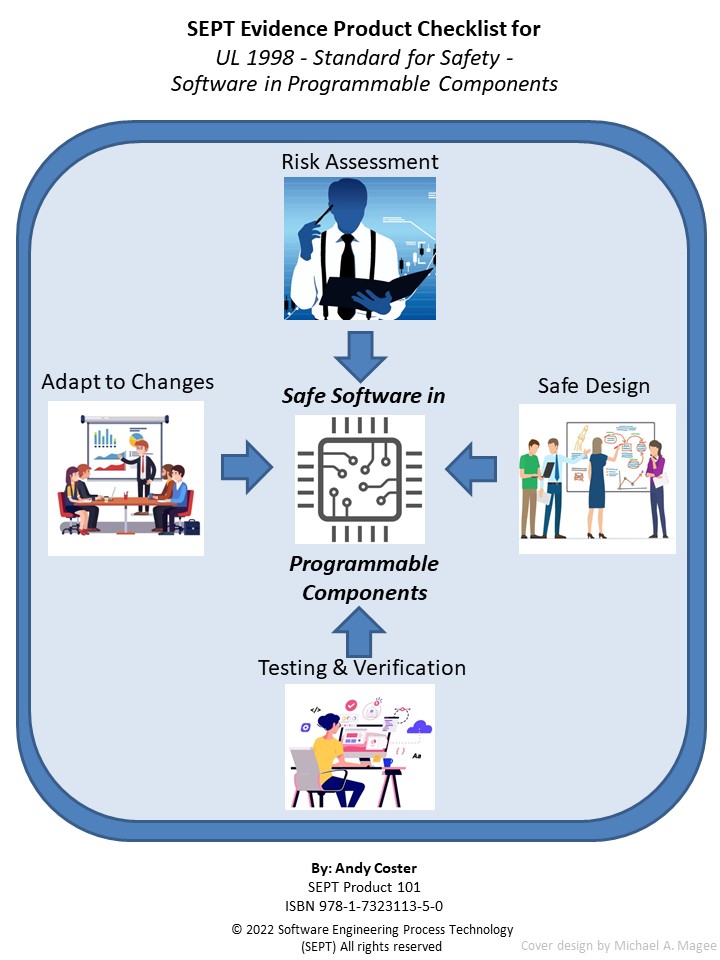
Price: $167 BUY NOW - Evidence Product Checklist For UL 1998 Standard for Safety - Software in Programmable Components
Price: $167 BUY NOW