Checklist for Standard ISO 9004:2018
Author: Andy Coster, CCP
Cover: Available
Customer Set for this product: All firms that would like an excellent quality program. SEPT is the only firm that makes such a checklist
Format: Word® (To save money, click here for our PDF version)
ISBN numbers: 978-1-7323113-0-5
Language: English
Page count: 241
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
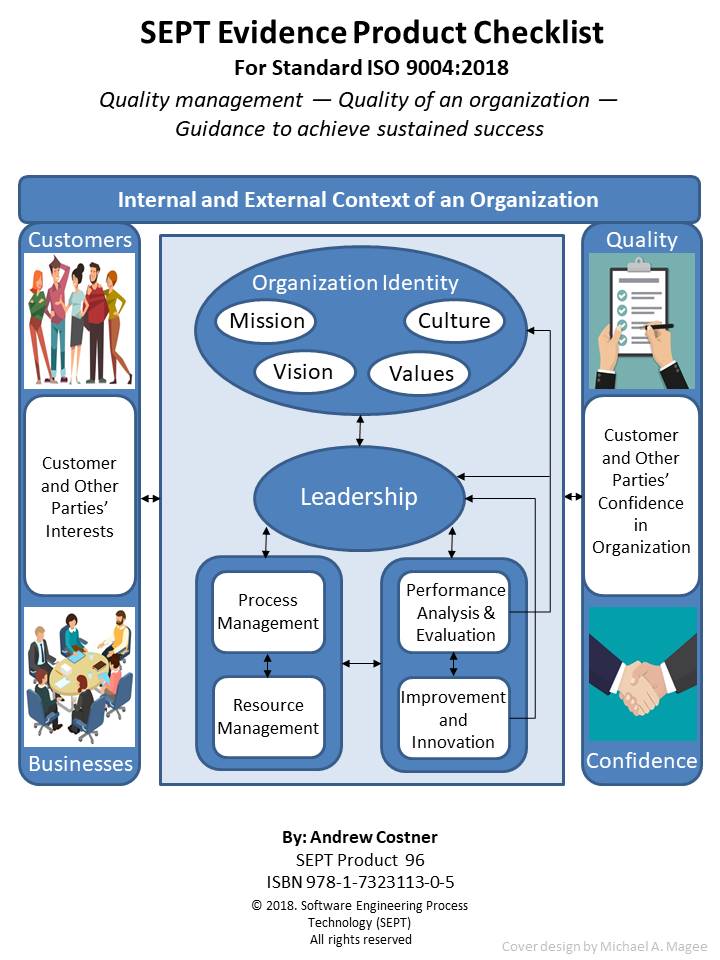
This checklist defines all the artifacts defined in the ISO 9004:2018 standard. It also assigns a maturity level to each artifact that can be used to assess the maturity level of an organization. In this “guidance” standard there are no “required” artefacts (no “shalls”) only “suggested” items.
For 20 + years Software Engineering Process Technology (SEPT) has produced checklists for standards that address software issues. To reduce the fog surrounding these types of standards SEPT has produced checklists for standards since 1994. This is another checklist related to quality standards.
ISO 9004 will provide your organization with guidance and support to achieve sustained success by using a quality management approach. This approach can be used by any organization, regardless of size, type and activity. To document that an organization has achieved a certain quality maturity level the standard recommends that the organization produce and use certain quality artifacts (Procedure, Policy, Plan, Records, Document, Audits and Review) at each maturity level. However, what constitutes physical evidence (Artifacts) to meet the guidance outlined in ISO 9004 is sometimes difficult to identify. To bridge this gap the author and SEPT experts have identified items of physical evidence called out in the standard based on their knowledge of the document and their experience in the quality field. Each item of physical evidence that was identified by these experts is listed in the checklist as; policy, procedure, plan, records, document or reviews.
ISO 9004 promotes self-assessment as an important tool for the review of the maturity level of an organization. This self-assessment can provide an overall view of the performance of an organization and degree of maturity of their management system. To aid in this transition from physical evidence to maturity level, the checklist identifies the maturity level associated with each piece of physical evidence called out in the checklist. Since the standard does not assign a maturity level to an item of physical evidence the author and SEPT experts have assigned a maturity level to each piece of physical evidence listed. The maturity level is marked in brackets () after the artefact in each checklist. This assigning of maturity level was based on the expert knowledge of the standard and their experience in the quality field.
The SEPT checklists are constructed around a classification scheme of physical evidence comprised of policies, procedures, plans, records, documents, audits, and reviews. There must be an accompanying record of some type when an audit or review has been accomplished. This record would define the findings of the review or audit and any corrective action to be taken. For the sake of brevity this checklist does not call out a separate record for each review or audit. All procedures should be reviewed but the checklist does not call out a review for each procedure, unless the standard calls out the procedure review. In this checklist, “manuals, reports, scripts and specifications” are included in the document category. In the procedure category, guidelines are included when the subject standard references another standard for physical evidence. The checklist does not call out the requirements of the referenced standard.
The checklist is available in PDF or word format. The latter format allows you to tailor the document to your business case or the media that your organization wants to use the checklist in such as excel web page format or any other end product type in order to meet compliance with the standard in the most efficient way possible.
The checklist comes with 4 hours of free consultation, from experts that have firsthand knowledge of the underlying standard, to answer questions on the standards and checklists and is valid for 60 days after purchase of the product.
This product supports these Software Engineering processes
- Documentation
- Quality
- Life Cycle
Customers of this product:
- Altaresources
- Cascade designs
- Dell
- Eaton Corp.
- Kydex, PLLC
- Lincoln composites
- MEO., Portugal
- Michael T. Jones & Company, PLLC
- Port Authority of New York and New Jersey
- QSDS
- Sasol Technology, South Africa
- Schneider Mills Inc
- Standard Car Truck Company
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- 13485 P-640 Work Environment
Price: $39 BUY NOW - 13485 P-710 Planning of Product Realization
Price: $39 BUY NOW - 13485 P-720 Customer Related Processes
Price: $39 BUY NOW - 13485 P-722 Risk Management
Price: $39 BUY NOW - 13485 P-730 Design and Development
Price: $39 BUY NOW - 13485 P-740 Purchasing
Price: $39 BUY NOW