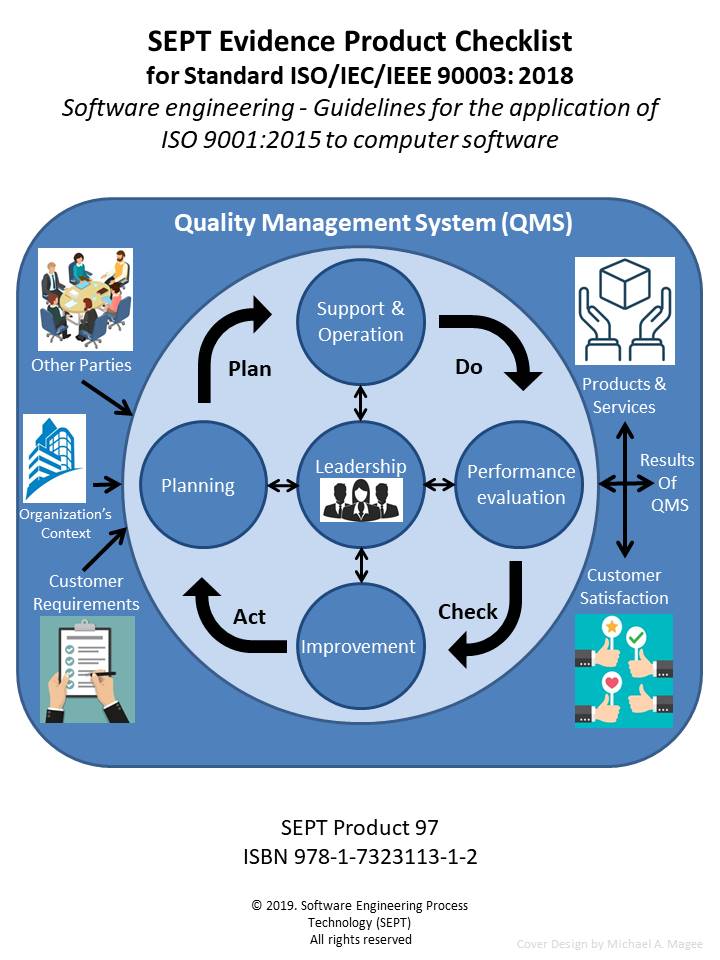
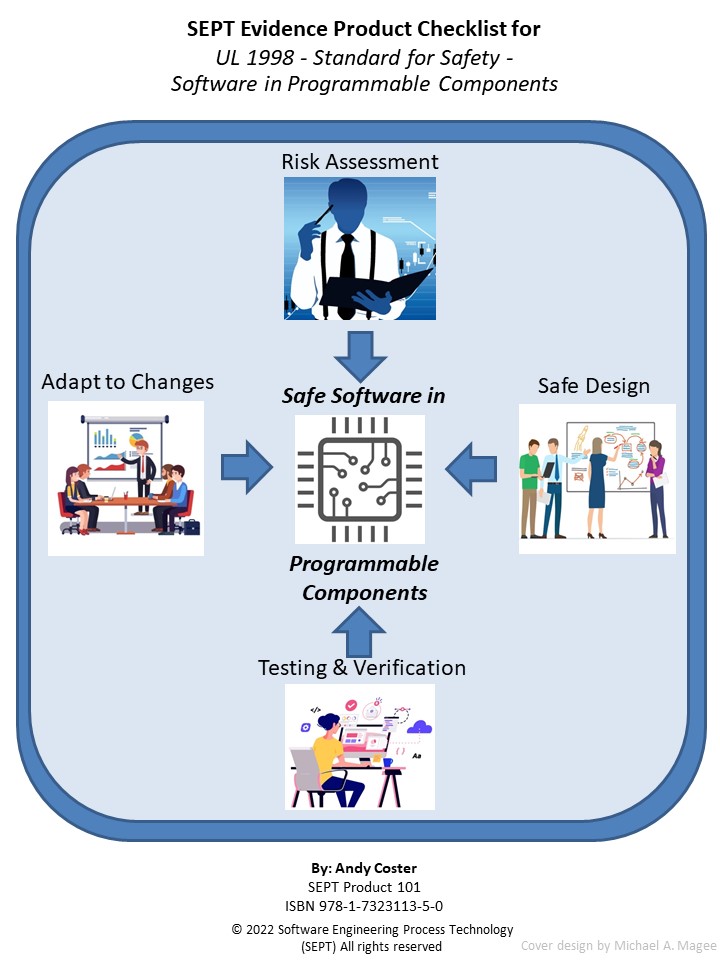
A Software Engineering Kit – Composed of Templates for Key Software Engineering Process Plans

Abstract: Available
Authors: Andy Coster, CCP, Stan Magee CCP (Ret.) John Neorr
Cover: Available
Customer Set for this product: Software engineering and system engineering firms and firms that have large and complex software or systems
Format: PDF (Click here for our easy-to-modify Word® formatted version)
Language: English
Page count of document: 400+
Provider: SEPT
Shipping: Available for download - Link will be provided in My ComplianceOnline section
Authors: Andy Coster, CCP, Stan Magee CCP (Ret.) John Neorr
Cover: Available
Customer Set for this product: Software engineering and system engineering firms and firms that have large and complex software or systems
Format: PDF (Click here for our easy-to-modify Word® formatted version)
Language: English
Page count of document: 400+
Provider: SEPT
Shipping: Available for download - Link will be provided in My ComplianceOnline section
Price:
$298.00
Product Details
SEPT customers asked for a kit containing templates for the most popular software and system process. These firms wanted templates that software professionals at all levels could use to produce effective, standards-compliant plans. After doing due diligence SEPT has selected the following templates to meet this need. This kit offers a savings of 50% off their individual template price.
This kit contains the following Software Engineering Templates- Template for a Software Maintenance Plan - Fifth Edition :Product Number 102.
- Software Documentation Management Plan Template - Second Edition :Product Number 55.
- System Documentation Management Plan Template - Second Edition :Product Number 59.
- Templates and Plans for Software Configuration Management Documents-Version 6.0 :Product Number 76.
This product supports these Software Engineering processes
- Acquisition
- Code and Test
- Configuration Management
- Design
- Documentation
- Integration
- Life Cycle
- Maintenance And Operation
- Metrics
- Process Improvements
- Project Management
- Quality
- Requirements Definition
- Safety
- Security
- Verification And Validation
Customers of this product:
- Chuoutokkyo Company, Japan
- American Opti Surgical Inc
- Barclays Global Investors
- Bio-Rad Laboratories
- Biosynthetic, Canda
- CONTRINEX SA
- DHSS
- distinction.co.nz
- EmWIB Technologies, India
- Haemonetics Corporation
- Integrated Systems Solutions
- Omnigon
- PV Labs Inc., Canada
- Salmat Business Force
- Seabrook, Ireland
- Shearwater Research Inc., Canada
- Skyllaeng
- Techmatrix Co, Japan
- Technica Corp
- Wagner-Biro Luxembourg Stage Systems S.A., Luxembourg
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- Configuration, Acronyms & Abbreviations in Technical Writing
Price: $25 BUY NOW - Checklist for Standard ISO/IEC 27002:2013 - Information Security Code of Practice
Price: $399 BUY NOW - System Documentation Management Plan Template- Second Edition
Price: $330 BUY NOW - Template for a Software Maintenance Plan - Fifth Edition
Price: $330 BUY NOW - Validation Master Plan Item Information Form (RiskVal)
Price: $99 BUY NOW - Software Documentation Management Plan Template - Second Edition
Price: $330 BUY NOW
You Recently Viewed