Electronic Signatures; Final Rule-FDA 21CFR Part 11
Author: Stan Magee CCP (Ret.)
Cover: Available
Customer Set for this product: Medical Device Firms
Format: PDF (Click here for our easy-to-modify Word® formatted version)
ISBN Numbers: 978-0-9716087-0-2 / ISBN 0-9716087-0-9
Language: English
Page count of document: 31
Provider: SEPT
Sample Pages: Available
Shipping: Available for download - Link will be provided in My ComplianceOnline section
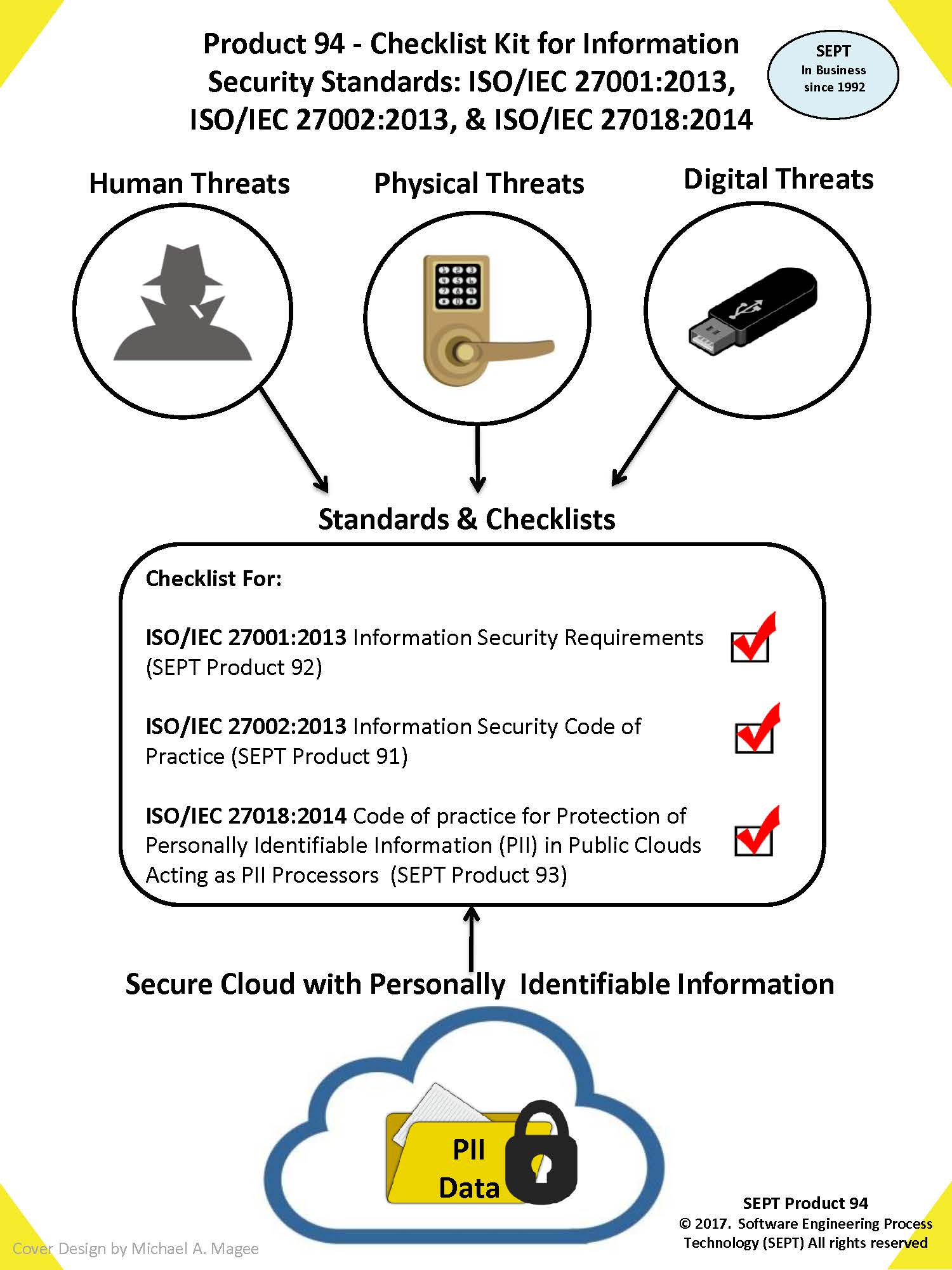
Checklist for the FDA Document : "FDA 21 CFR Part 11 - Electronic Records; Electronic Signatures; Final Rule".
This checklist clarifies what is required for compliance to this standard by providing an easy-to-use product evidence list that will assist any software organization in meeting the requirements of this standard.
The Checklist uses a classification scheme of physical evidence comprised of
- Procedure
- Plan
- Records
- Document
- Review
- Audit
When a company is planning to use this document to ensure their compliance to FDA 21 CFR Part 11, the company should review this evidence checklist.
This product supports these Software Engineering processes
- Acquisition
- Documentation
- Quality Assurance
- Safety
Customers of this product:
- Agilent Technologies
- Auxogyn, Inc.
- B&C Calibration Center Inc., Puerto Rico
- Baxter Healthcare
- Daiichi Asabi Pharmaceuticals
- Windy Hill Medical, Inc
- Environmental Systems Corporation
- Key Technology Inc.
- Lumina Engineering
- Miquest Limited
- Precision Interconnect
- Profit Technologies
- Sandvik
- Shiseido
- Sypris T&M
- Testo GmbH & Co., Germany
- Uptake Medical
- Vapotherm
- Veracity Medical Solutions
Note: “International Standards (ISO) define the best of practices for Medical Device and Software firms in producing a quality product. This checklist that SEPT produces will ensure that all of the best of practices are adhered to.”
Customers Also Bought
- ISO 13485:2016 “Medical Devices - Quality Management Systems- Requirements for Regulatory Purposes”
Price: $167 BUY NOW - Evidence Product Checklist For UL 1998 Standard for Safety - Software in Programmable Components
Price: $167 BUY NOW - ISO 17025 Quality Manual Template
Price: $299 BUY NOW - Requirements for Computer System Requirements, Validation Plans, Protocols and Reports (RiskVal)
Price: $99 BUY NOW - Change Control for Validated Systems (RiskVal)
Price: $125 BUY NOW - IT Policy (RiskVal)
Price: $99 BUY NOW